Control of Muscle Activity by Ca2+
This is an excerpt from Exercise Biochemistry 2nd Edition by Vassilis Mougios.
For more than 130 years (since 1883), it has been known that muscles cannot contract in the absence of calcium cations. However, 80 years had to pass before the exact role of Ca2+ was discovered. Today we know that Ca2+ controls muscle activity and that it does so by permitting the binding of myosin to F-actin. This action of Ca2+ is not direct but indirect. As the Japanese physiologist Setsuro Ebashi discovered in the 1960s, control is exerted through tropomyosin and troponin, the two proteins that coexist with actin in the thin filaments, constituting about one third of the thin-filament mass. Let's meet them.
Tropomyosin has a molecular mass of 70 kDa and consists of two similar stringlike subunits in -helical conformation. The two subunits wind around each other just as the myosin heavy chains do in the myosin tail. The resulting extremely long tropomyosin molecules join in a row to form fibers. Two such fibers run along each thin filament while following the twisting of the actin monomers (figure 8.11).
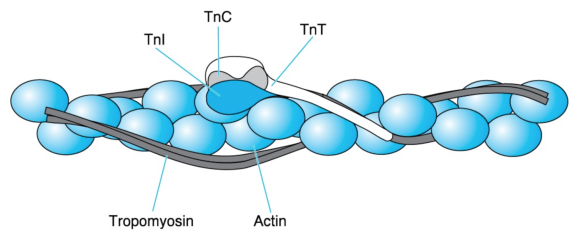
Figure 8.11 Thin-filament proteins. A thin filament consists of an F-actin fiber (the double necklace of figure 8.7), two series of tropomyosin molecules, and troponin (Tn) complexes placed at regular intervals. Each troponin complex consists of TnC, which is the Ca2+ acceptor; TnI, which binds to actin; and TnT, which extends by way of a long tail along tropomyosin.
Adapted from L. Smillie and S. Ebashi, Essays in Biochemistry, vol. 10, edited by P.N. Campbell and F. Dickens (Orlando, FL: Academic, 1974), 1-35; C. Cohen Scientific America 233 (1975): 36 - 45. Courtesy of L. Smillie.
Troponin, symbolized as Tn, is a complex of three different subunits: TnC (18 kDa), TnI (24 kDa), and TnT (37 kDa). TnC binds Ca2+, TnI binds to actin, and TnT binds to tropomyosin. Two troponin complexes appear on the two sides of a thin filament every 39 nm, which is approximately the length of a tropomyosin molecule. One troponin complex attached to one tropomyosin molecule controls approximately seven actin monomers.
When a muscle is at rest (relaxed), the cytosol has a very low [Ca2+], approximately 10-7 mol · L-1. Researchers believe that, in this case, interactions among F-actin, TnI, TnT, and tropomyosin hold the latter close to the sites on the actin monomers where the myosin heads bind. Thus, tropomyosin hinders the interaction of thin and thick filaments. As we will see in the next section, muscle excitation by the nervous system results in the release of Ca2+ from an intracellular reservoir called the sarcoplasmic reticulum. This release causes a 100-fold surge in the cytosolic [Ca2+], from 10-7 to 10-5 mol · L-1.
The increased Ca2+ ions encounter TnC, bind to it, and elicit a change in its conformation. As a result, TnC detaches TnI from F-actin. This detachment lets tropomyosin move over the surface of the thin filament, away from the binding sites of myosin on F-actin. Myosin then binds to F-actin, and the muscle contracts. In fact, there is evidence that myosin binding to F-actin is cooperative; that is, the binding of some myosin heads facilitates the binding of additional ones by promoting the displacement of tropomyosin. This effect is reminiscent of the cooperative binding of O2 to hemoglobin (section 3.12).
Muscle activity continues until Ca2+ is sequestered in the sarcoplasmic reticulum (in a manner that we will examine shortly). The actin-TnI-TnT-tropomyosin interaction is then restored. Tropomyosin returns to a position hindering cross-bridge formation, and the muscle relaxes. The chain of events through which Ca2+ controls muscle contraction is summarized in figure 8.12.
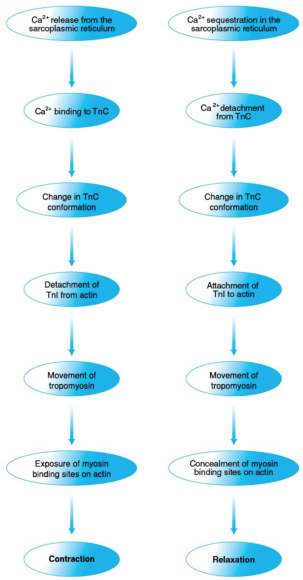
Figure 8.12 Control of muscle contraction and relaxation by Ca2+. Ca2+ controls muscle contraction (left) and relaxation (right) through a series of protein interactions triggered by, respectively, Ca2+ release from and sequestration in the sarcoplasmic reticulum and involving troponin, tropomyosin, actin, and myosin.
More Excerpts From Exercise Biochemistry 2nd EditionSHOP

Get the latest insights with regular newsletters, plus periodic product information and special insider offers.
JOIN NOW
Latest Posts
- Women in sport and sport marketing
- Sport’s role in the climate crisis
- What international competencies do sport managers need?
- Using artificial intelligence in athletic training
- Using the evidence pyramid to assess athletic training research
- How can athletic trainers ask a clinically relevant question using PICO?


