Adaptations of the Vasculature to Training
This is an excerpt from Exercise Biochemistry 2nd Edition by Vassilis Mougios.
Training benefits the blood vessels as well as the heart. As discussed in sections 11.21 and 11.22, endurance and resistance training can lower the risk of atherosclerosis by decreasing plasma triacylglycerols, total cholesterol, and LDL cholesterol. On top of these effects, endurance training contributes to lowering the risk of atherosclerosis by increasing HDL cholesterol.
In addition, the increases in blood flow and pressure accompanying exercise cause structural and functional adaptations of the vascular wall that lower the risk of atherosclerosis. These beneficial effects seem to be mediated by the endothelium, the single layer of cells lining the interior surface of the blood vessels, in direct contact with blood on the inside and surrounded by smooth muscle cells on the outside (figure 15.3).
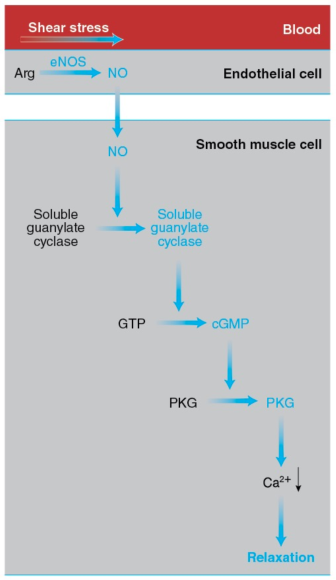
Figure 15.3 How exercise elicits vasodilation. The increase in the pumping activity of the heart during exercise augments the shear stress on the endothelial cells lining the blood vessels. This mechanical stimulus leads to activation of eNOS, which catalyzes the synthesis of NO from arginine. NO diffuses to the smooth muscle cells forming the walls of blood vessels and activates soluble guanylate cyclase, which catalyzes the synthesis of cyclic GMP (cGMP) from GTP. Cyclic GMP activates PKG, leading to a drop in the cytosolic Ca2+ concentration, relaxation of the smooth muscle cells, and vasodilation.
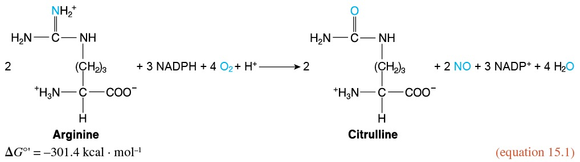
The endothelial cells possess a variety of proteins that convert the mechanical stimulus of the exercise-induced increase in shear stress into chemical signals. In turn, these signals activate endothelial nitrogen oxide synthase (eNOS). This enzyme catalyzes the synthesis of nitric oxide (introduced in section 1.5) from arginine and oxygen in a reaction requiring NADPH as a reductant and yielding citrulline (the intermediate compound of the urea cycle introduced in section 12.9 and figure 12.10) in addition to NO.
NO diffuses out of the endothelial cells and enters neighboring smooth muscle cells, where it activates soluble guanylate cyclase, a cytosolic enzyme that catalyzes the formation of cyclic guanylate, or cyclic GMP (cGMP), from GTP, in a manner analogous to the synthesis of cAMP (section 10.6 and reaction 10.5).
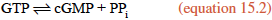
Cyclic GMP, in turn, activates cGMP-dependent protein kinase, or PKG, which promotes smooth muscle relaxation by activating an ion pump that removes Ca2+ from the cytosol, thus preventing the interaction of myosin and actin. (Ca2+ allows myosin binding to actin in smooth muscle cells and skeletal muscle fibers through a series of interactions differing from the one described in section 8.10.) Smooth muscle relaxation results in dilation of the blood vessels, or vasodilation. The capacity of the vessels to dilate in response to increased blood flow, a property termed flow-mediated dilation, is considered an index of cardiovascular health.
As Green and coworkers review, training improves flow-mediated dilation—especially in individuals with or at risk of CVD—and reduces arterial wall stiffness by enhancing the endothelium-dependent signal transduction pathway described earlier. The enhancement includes higher eNOS content or activity. It is also possible that training influences other pathways of flow-mediated dilation. In addition, training increases the diameter of vessels—such as the coronary arteries, as well as arteries that nourish the exercising limbs—thus supplying more blood where it is needed. The stimulus, again, appears to be the increased shear stress imposed by blood on the endothelium with exercise.
Finally, training influences the smaller blood vessels: It increases the amount and diameter of the arterioles (the branches of arteries leading to capillaries) and muscle capillarization. The latter is measured as either the total number of capillaries in a muscle, or the number of capillaries per muscle fiber, or capillary density (defined in section 14.15 as the number of capillaries per unit of muscle cross-sectional area). Increased capillarization enhances the delivery of nutrients and O2 to muscle, as well as the uptake of muscle CO2 by blood.
Training-induced capillarization may be due to increased blood flow or to muscle activity itself. Both factors may promote the release of vascular endothelial growth factor (VEGF), a key angiogenic (that is, vessel-generating) protein, from muscle fibers. VEGF binds to the VEGF receptor in the plasma membrane of endothelial cells and activates a variety of signal transduction pathways, leading to endothelial cell proliferation and, hence, formation of new capillaries.
You can see that training provides more than one way of increasing blood supply to the active muscles. It seems that these beneficial adaptations can be elicited by endurance, resistance, and interval training. Improvements in vascular function with training are evident in both healthy humans and (possibly more evident) humans with or at risk of CVD.
SHOP

Get the latest insights with regular newsletters, plus periodic product information and special insider offers.
JOIN NOW
Latest Posts
- Women in sport and sport marketing
- Sport’s role in the climate crisis
- What international competencies do sport managers need?
- Using artificial intelligence in athletic training
- Using the evidence pyramid to assess athletic training research
- How can athletic trainers ask a clinically relevant question using PICO?


